Ammonia
|
|
| Ammonia | |
|---|---|
| |
| General | |
| Systematic name | Ammonia |
| Trivial names | Spirit of hartshorn, nitrosil, Vaporole |
| Molecular formula | NH3 |
| Molar mass | 17.03 g/mol |
| Appearance | Colourless gas with strong pungent odor |
| CAS number | [7664-41-7] |
| Properties | |
| Density and phase | 0.7714 g/cm3, gas |
| Solubility in water | 54 g/100 ml |
| Melting point | -78 °C (195 K) |
| Boiling point | -33 °C (240 K) |
| Basicity (pKb) | 4.75 |
| Acidity (pKa) | ? very weak |
| Structure | |
| Molecular shape | trigonal pyramidal |
| Dipole moment | ? D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | flammable, reactive |
| Flash point | 11 °C |
| R/S statement | R: 10, 23, 34, 50 S: 1/2, 9, 16, 26, 36/37/39, 45, 61 |
| RTECS number | BO0875000 |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related Amines | See Amine |
| Related compounds | Hydrazine (N2H4) |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references | |
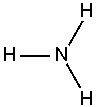
Ammonia is a chemical compound with the formula NH3, since its molecule has a nitrogen atom with three hydrogen atoms all singly covalently bonded to the nitrogen as shown in the image at right. At standard temperature and pressure, ammonia is a gas. This gas is toxic and corrosive to some materials and has a characteristic pungent odor.
An ammonia molecule is not flat, but has the shape of a flattened tetrahedron known as a trigonal pyramid. This shape gives the molecule an overall dipole moment and makes it polar so that ammonia very readily dissolves in water. The nitrogen atom in the molecule has a lone electron pair; therefore, ammonia acts as a base. In acidic or even aqueous solutions, it can bond to an H+ ion to form the positively charged ammonium ion NH4+, which has the shape of a regular tetrahedron. The degree to which ammonia forms the ammonium ion depends on its concentration and the pH of the solution.
The main uses of ammonia are in the production of fertilizers, explosives and polymers, but it is probably most familiar as an ingredient in household glass cleaners. Ammonia is found in small quantities as the ammonium carbonate in the atmosphere, being produced from the putrefaction of nitrogenous animal and vegetable matter. Ammonium salts are also found in small quantities in rain-water, while ammonium chloride (sal-ammoniac) and ammonium sulfate are found in volcanic districts; and crystals of ammonium bicarbonate have been found in Patagonian guano. Ammonium salts also are found distributed through all fertile soil, in sea-water, and in most plant and animal liquids, and also in urine.
| Contents |
History
Salts of ammonia have been known from very early times; thus the term Hammoniacus sal appears in the writings of Pliny, although it is not known whether the term is identical with the more modern sal-ammoniac.
In the form of sal-ammoniac, ammonia was known, however, to the alchemists as early as the 13th century, being mentioned by Albertus Magnus, while in the 15th century Basil Valentine showed that ammonia could be obtained by the action of alkalies on sal-ammoniac. At a later period when sal-ammoniac was obtained by distilling the hoofs and horns of oxen, and neutralizing the resulting carbonate with hydrochloric acid, the name spirits of hartshorn was applied to ammonia.
Gaseous ammonia was first isolated by Joseph Priestley in 1774 and was termed by him alkaline air. In 1777 Karl Wilhelm Scheele showed that it contained nitrogen, and Claude Louis Berthollet, in about 1785, ascertained its composition.
The Haber process to produce ammonia from the nitrogen contained in the air was developed by Fritz Haber and Carl Bosch in 1909 and patented in 1910. It was first used on an industrial scale by the Germans during WWI. The ammonia was used to produce explosives to sustain their war effort.
Production
Because of its many uses, ammonia is one of the most highly-produced inorganic chemicals. Before the start of WWI most ammonia was obtained by the dry distillation of nitrogenous vegetable and animal products; by the reduction of nitrous acid and nitrites with nascent hydrogen; and also by the decomposition of ammonium salts by alkaline hydroxides or by unslaked lime (quicklime), the salt most generally used being the chloride (sal-ammoniac) thus
A similar reaction yields
- 2NH4Cl + CaO → CaCl2 + H2O +2NH3
It was also obtained by decomposing magnesium nitride (Mg3N2) with water,
- Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
Today the Haber process is the most important method for production of ammonia. In this process, nitrogen and hydrogen gases combine directly on an iron catalyst at high pressure of 3000 lbf/in² (20 MPa) and temperature (500 °C) to produce ammonia.
Compared to older methods, the Haber process's feedstocks are relatively inexpensive—nitrogen makes up 78% of the atmosphere, while hydrogen can be readily produced from natural gas.
Properties
Ammonia is a colourless gas possessing a characteristic pungent smell and a strongly alkaline reaction; it is lighter than air, its density being 0.589 times that of air. It is easily liquefied and the liquid boils at -33.7 °C, and solidifies at -75 °C to a mass of white crystals. Liquid ammonia possesses strong ionizing powers, and solutions of salts in liquid ammonia have been much studied. Liquid ammonia has a very high specific heat capacity and can therefore be used in laboratories in non-insulated vessels at room temperature, even though it is well above its boiling point.
It is miscible with water. All the ammonia contained in an aqueous solution of the gas may be expelled by boiling. The aqueous solution of ammonia is very basic, and since it is a weak electrolyte, the solution will contain a small amount of ammonium hydroxide (NH4OH). The maximum concentration of ammonia in water (a saturated solution) has a density of 880 kg m-3 and is often known as '880 Ammonia'.
It does not support combustion, and it does not burn readily unless mixed with oxygen, when it burns with a pale yellowish-green flame. However it can form an explosive mixture with air.
An ammonia molecule readily undergoes nitrogen inversion.
Detection
Ammonia and ammonium salts can be readily detected, in very minute traces, by the addition of Nessler's solution, which gives a distinct yellow coloration in the presence of the least trace of ammonia or ammonium salts. Larger quantities can be detected by warming the salts with a caustic alkali or with quicklime, when the characteristic smell of ammonia will be at once apparent. The amount of ammonia in ammonium salts can be estimated quantitatively by distillation of the salts with sodium or potassium hydroxide, the ammonia evolved being absorbed in a known volume of standard sulfuric acid and the excess of acid then determined volumetrically; or the ammonia may be absorbed in hydrochloric acid and the ammonium chloride so formed precipitated as ammonium chlorplatinate, (NH4)2PtCl6.
Uses
In addition to serving as a fertilizer ingredient, ammonia can also be used directly as a fertilizer by forming a solution with irrigation water, without additional chemical processing. This later use allows the continuous growing of nitrogen dependent crops such as maize (corn) without crop rotation but this type of use leads to poor soil health.
Ammonia has thermodynamic properties that make it very well suited as a refrigerant, since it liquefies readily under pressure, and was used in virtually all refrigeration units prior to the advent of haloalkanes such as Freon. However, ammonia is a toxic irritant and its corrosiveness to any copper alloys increases the risk that an undesirable leak may develop and cause an obnoxious hazard. It's use in small refrigeration units has been largely replaced by haloalkanes, which are not toxic irritants and are practically not flammable. (Note: Butane and isobutane, which have very suitable thermodynamic properties for refrigerants, are extremely flammable.) Ammonia continues to be used as a refrigerant in large industrial processes such as bulk icemaking and industrial food processing. Ammonia is also useful as a component in absorption-type refrigerators, which do not use compression and expansion cycles but can exploit heat differences. Since the implication of haloalkane being major contributors to ozone depletion, ammonia is again seeing increasing use as a refrigerant.
Ammonia is a primary ingredient in old-style household cleaners.
It is also an ingredient in the process of chloramination for disinfection of drinking water supplies. Unlike the use of gaseous chlorine for this purpose it does not combine with organic (carbon containing) materials to form halomethanes such as carbon tetrachloride, which is a long–term hazard to human health, even in minuscule concentrations, as it is considered to be carcinogenic.
Formation of salts
One of the most characteristic properties of ammonia is its power of combining directly with acids to form salts; thus with hydrochloric acid it forms ammonium chloride (sal-ammoniac); with nitric acid, ammonium nitrate, etc. It is to be noted that H. B. Baker (Journal of Chem. Soc., 1894, lxv. p. 612) has shown that perfectly dry ammonia will not combine with perfectly dry hydrogen chloride, moisture being necessary to bring about the reaction.
The salts produced by the action of ammonia on acids are known as the ammonium salts and all contain the compound radical ammonium (NH4+). Numerous attempts have been made to isolate this radical, but so far none have been successful. By the addition of sodium amalgam to a concentrated solution of ammonium chloride, the so-called ammonium amalgam is obtained as a spongy mass which floats on the surface of the liquid; it decomposes readily at ordinary temperatures into ammonia and hydrogen; it does not reduce silver and gold salts, a behaviour which distinguishes it from the amalgams of the alkali metals, and for this reason it is regarded by some chemists as being merely mercury inflated by gaseous ammonia and hydrogen. M. le Blanc has shown, however, that the effect of ammonium amalgam on the magnitude of polarization of a battery is comparable with that of the amalgams of the alkali metals.
Formation of other compounds
Ammonia finds a wide application in organic chemistry as a synthetic reagent; it reacts with alkyl iodides to form amines, with esters to form acid amides, with halogen fatty acids to form amino acids; while it also combines with isocyanic esters to form alkyl ureas and with the mustard oils to form alkyl thioureas. Aldehydes also combine directly with ammonia.
Ammonia gas has the power of combining with many substances, particularly with metallic halides; thus with calcium chloride it forms the compound CaCl2•8NH3, and consequently calcium chloride cannot be used for drying the gas. With silver chloride it forms two compounds -- one, AgCl•3NH3 at temperatures below 15 °C; the other, 2AgCl•3NH3 at temperatures above 20 °C. On heating these substances, ammonia is liberated and the metallic chloride remains. It was by the use of silver chloride ammonia compounds that in 1823 Michael Faraday was first able to liquefy ammonia. It can be shown by Isambert's results that the compound AgCl•3NH3 cannot be formed above 20 °C, by the action of ammonia on silver chloride at atmospheric pressure; while 2AgCl•3NH3, under similar conditions, cannot be formed above about 68 °C.
With iodine it reacts to form nitrogen iodide. This compound was discovered in 1812 by Bernard Courtois, and was originally supposed to contain nitrogen and iodine only, but in 1840 R.F. Marchand showed that it contained hydrogen, while R. Bunsen showed that no oxygen was present. As regards its constitution, it has been given at different times the formulae NI3, NHI2, NH2I, N2H3I3, etc., these varying results being due to the impurities in the substance, owing to the different investigators working under unsuitable conditions, and also to the decomposing action of light. F. D. Chattaway determined its composition as N2H3I3, by the addition of excess of standard sodium sulfite solution, in the dark, and subsequent titration of the excess of the sulfite with standard iodine. The constitution has been definitely determined by O. Silberrad (Jour. of Chem. Soc., 1905, lxxxvii. p. 55) by the interaction of nitrogen iodide with zinc ethyl, the products of the reaction being triethylamine and ammonia; the ammonia liberated was absorbed in hydrochloric acid, and 95% of the theoretical amount of the ammonium chloride was obtained. On these grounds O. Silberrad assigns the formula NH3•NI3 to the compound, and explains the decomposition as taking place,
- 2 NH3•NI3 + 6 Zn(C2H5)2 → 6 ZnC2H5•I + 2 NH3 + 2 N(C2H5)3.
The hydrogen in ammonia is capable of replacement by metals, thus magnesium burns in the gas with the formation of magnesium nitride Mg3N2, and when the gas is passed over heated sodium or potassium, sodamide, NaNH2, and potassamide, KNH2, are formed.
Liquid ammonia as a solvent
Liquid ammonia is used for the artificial preparation of ice. It readily dissolves sodium and potassium, giving in each case a dark blue solution. At a red heat ammonia is easily decomposed into its constituent elements, a similar decomposition being brought about by the passage of electric sparks through the gas. Chlorine takes fire when passed into ammonia, nitrogen, and hydrochloric acid being formed, and unless the ammonia be present in excess, the highly explosive nitrogen trichloride NCl3 is also produced.
Liquid ammonia is the best-known and most widely studied non-aqueous ionizing solvent. Its most conspicuous property is its ability to dissolve alkali metals to form highly coloured, electrically conducting solutions containing solvated electrons. Apart from these remarkable solutions, much of the chemistry in liquid ammonia can be classified by analogy with related reactions in aqueous solutions. Comparison of the physical properties of NH3 with those of water shows that NH3 has the lower melting point, boiling point, density, viscosity, dielectric constant and electrical conductivity; this is due at least in part to the weaker H bonding in NH3 and the fact that such bonding cannot form cross-linked networks since each NH3 molecule has only 1 lone-pair of electrons compared with 2 for each H2O molecule. The ionic self-dissociation constant of liquid NH3 at −50 °C is approx. 10-33 mol2·l-2.
Hazards
As both ammonia in water solution and chlorine bleach (sodium hypochlorite in water solution) are common household cleaners there is considerable danger that these may be used in combination in order to obtain a more active cleaning agent. This is extremely dangerous as the combination of the two solutions will react to form chloramines: monochloramine NH2Cl, dichloramine NHCl2 and trichloramine (nitrogen trichloride) NCl3). Nitrogen trichloride in its pure form is an highly unstable (explosive!), oily liquid. Nitrogen trichloride rapidly decomposes and releases toxic Cl2 (chlorine), gas.
References
- Data on the heat of fusion and heat of vaporization are from The Planetary Scientist's Companion, by Katharina Lodders and Bruce Fegley, Jr. (New York: Oxford UP Inc., 1998).cs:Čpavek
cy:Amonia da:Ammoniak de:Ammoniak es:Amoníaco fr:Ammoniac io:Amoniako it:Ammoniaca he:אמוניה ms:Ammonia nl:Ammoniak no:Ammoniakk nn:Ammoniakk ja:アンモニア pl:Amoniak pt:Amoníaco ru:Аммиак simple:Ammonia fi:Ammoniakki sv:Ammoniak zh:氨
