Aniline
|
|
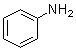
Aniline, phenylamine or aminobenzene (C6H5NH2) is an organic chemical compound which is a primary aromatic amine consisting of a benzene ring and an amino group. The chemical structure of aniline is shown at the right. It is a liquid at room temperature.
| Contents |
Synthesis
Aniline can be produced from benzene in two steps. First, benzene is nitrated (reacted with nitric acid, a form of electrophilic substitution reaction) to give nitrobenzene. Second, the nitrobenzene is reduced to give aniline. A variety of reducing agents are effective for the reduction, including H2 (with a catalyst), hydrogen sulfide, iron, zinc, or tin.
Many derivatives of aniline can be prepared similarly.
Properties
Aniline is a carcinogen. It is oily and although colourless, it can be slowly oxidized and resinified in air to form impurities which can give it a red-brown tint. Its boiling point is 184 °C and its melting point is -6 °C.
It ignites readily, burning with a large smoky flame. It possesses a somewhat pleasant vinous odour and a burning aromatic taste; it is a highly acrid poison.
Chemically, aniline is a weak base. Aromatic amines such as aniline are generally much weaker bases than aliphatic amines. Aniline reacts with strong acids to form salts containing the anilinium ion (C6H5-NH3+), and reacts with acyl halides (such as acetyl chloride, CH3COCl) to form amides. The amides formed from aniline are sometimes called anilides, for example CH3-CO-NH-C6H5 is acetanilide.
The sulphate forms beautiful white plates. Although aniline is but feebly basic, it precipitates zinc, aluminium and ferric salts, and on warming expels ammonia from its salts. Aniline combines directly with alkyl iodides to form secondary and tertiary amines; boiled with carbon disulphide it gives sulphocarbanilide (diphenyl thio-urea), CS(NHC6H5)2, which may be decomposed into phenyl mustard-oil, C6H5CNS, and triphenyl guanidine, C6H5N: C(NHC6H5)2. Sulphuric acid at 180° gives sulphanilic acid, NH2.C6H4.SO3H. Anilides, compounds in which the amino group is substituted by an acid radical, are prepared by heating aniline with certain acids; antifebrin or acetanilide is thus obtained from acetic acid and aniline. The oxidation of aniline has been carefully investigated. In alkaline solution azobenzene results, while arsenic acid produces the violet-colouring matter violaniline. Chromic acid converts it into quinone, while chlorates, in the presence of certain metallic salts (especially of vanadium), give aniline black. Hydrochloric acid and potassium chlorate give chloranil. Potassium permanganate in neutral solution oxidizes it to nitrobenzene, in alkaline solution to azobenzene, ammonia and oxalic acid, in acid solution to aniline black. Hypochlorous acid gives para-amino phenol and para-amino diphenylamine
Like phenols, aniline derivatives are highly reactive in electrophilic substitution reactions. For example, sulfonation of aniline produces sulfanilic acid, which can be converted to sulfanilamide. Sulfanilamide is one of the sulfa drugs which were widely used as antibacterials in the early 20th century.
Aniline and its ring-substituted derivatives react with nitrous acid to form diazonium ions. Through these, the -NH2 group of aniline can be conveniently converted to -OH, -CN, or a halide.
Uses
The great commercial value of aniline is due to the readiness with which it yields, directly or indirectly, valuable dyestuffs. The discovery of mauve in 1858 by William Perkin was the first of a series of dyestuffs which are now to be numbered by hundreds. Reference should be made to the articles dyeing, fuchsine, safranine, indulines, for more details on this subject. In addition to dyestuffs, it is a starting-product for the manufacture of many drugs, such as antipyrine, antifebrin, etc. Aniline is manufactured by reducing nitrobenzene with iron and hydrochloric acid and steam-distilling the product. The purity of the product depends upon the quality of the benzene from which the nitrobenzene was prepared. In commerce three brands of aniline are distinguished—aniline oil for blue, which is pure aniline; aniline oil for red, a mixture of equimolecular quantities of aniline and ortho- and para-toluidines; and aniline oil for safranine, which contains aniline and ortho-toluidine, and is obtained from the distillate (échappés) of the fuchsine fusion. Monomethyl and dimethyl aniline are colourless liquids prepared by heating aniline, aniline hydro-chloride and methyl alcohol in an autoclave at 220°. They are of great importance in the colour industry. Monomethyl aniline boils at 193-195°; dimethyl aniline at 192°.
History
Aniline was first isolated from the destructive distillation of indigo in 1826 by Otto Unverdorben (Pogg. Ann., 1826, 8, p. 397), who named it crystalline. In 1834, F. Runge (Pogg. Ann., 1834, 31, p. 65; 32, p. 331) isolated from coal tar a substance which produced a beautiful blue colour on treatment with chloride of lime; this he named kyanol or cyanol. In 1841, C. J. Fritzsche showed that by treating indigo with caustic potash it yielded an oil, which he named aniline, from the specific name of one of the indigo-yielding plants, Indigofera anil, anil being derived from the Sanskrit nīla, dark-blue, and nīlā, the indigo plant. About the same time N. N. Zinin found that on reducing nitrobenzene, a base was formed which he named benzidam. August Wilhelm von Hofmann investigated these variously prepared substances, and proved them to be identical (1855), and thenceforth they took their place as one body, under the name aniline or phenylamine.
Its first industrial-scale use was in the manufacture of mauveine, a purple dye discovered in 1856 by William Henry Perkin.
Controversy
Oil mixtures containing rapeseed oil denatured with aniline have been clearly linked by epidemiological and analytic chemical studies to the toxic oil syndrome that hit Spain in the spring and summer of 1981, in which 20,000 became acutely ill, 12,000 were hospitalized, and more than 350 died in the first year of the epidemic. The precise etiology though remains unknown.