Antimony
|
|
| |||||||||||||||||||||||||
| General | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
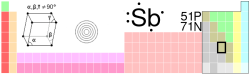
| Name, Symbol, Number | antimony, Sb, 51 | ||||||||||||||||||||||||
| Series | metalloids | ||||||||||||||||||||||||
| Group, Period, Block | 15 (VA), 5, p | ||||||||||||||||||||||||
| Density, Hardness | 6697 kg/m3, 3 | ||||||||||||||||||||||||
| Appearance | silvery lustrous grey
| ||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||
| Atomic weight | 121.760 amu | ||||||||||||||||||||||||
| Atomic radius (calc.) | 145 (133) pm | ||||||||||||||||||||||||
| Covalent radius | 138 pm | ||||||||||||||||||||||||
| van der Waals radius | no data | ||||||||||||||||||||||||
| Electron configuration | [Kr]4d10 5s2 5p3 | ||||||||||||||||||||||||
| e- 's per energy level | 2, 8, 18, 18, 5 | ||||||||||||||||||||||||
| Oxidation states (Oxide) | ?3, 5 (mildly acidic) | ||||||||||||||||||||||||
| Crystal structure | Rhombohedral | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| State of matter | Solid | ||||||||||||||||||||||||
| Melting point | 903.78 K (1167.13 ?F) | ||||||||||||||||||||||||
| Boiling point | 1860 K (2889 ?F) | ||||||||||||||||||||||||
| Molar volume | 18.19 ×10-6 m3/mol | ||||||||||||||||||||||||
| Heat of vaporization | 77.14 kJ/mol | ||||||||||||||||||||||||
| Heat of fusion | 19.87 kJ/mol | ||||||||||||||||||||||||
| Vapor pressure | 2.49 E-9 Pa @ 6304 K | ||||||||||||||||||||||||
| Speed of sound | __ m/s at __ K | ||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||
| Electronegativity | 2.05 (Pauling scale) | ||||||||||||||||||||||||
| Specific heat capacity | 210 J/(kg*K) | ||||||||||||||||||||||||
| Electrical conductivity | 2.88 106/(m?ohm) | ||||||||||||||||||||||||
| Thermal conductivity | 24.3 W/(m*K) | ||||||||||||||||||||||||
| 1st ionization potential | 834 kJ/mol | ||||||||||||||||||||||||
| 2nd ionization potential | 1594.9 kJ/mol | ||||||||||||||||||||||||
| 3rd ionization potential | 2440 kJ/mol | ||||||||||||||||||||||||
| 4th ionization potential | 4260 kJ/mol | ||||||||||||||||||||||||
| 5th ionization potential | 5400 kJ/mol | ||||||||||||||||||||||||
| 6th ionization potential | 10400 kJ/mol | ||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SI units & STP are used except where noted. | |||||||||||||||||||||||||
Antimony is a chemical element in the periodic table that has the symbol Sb (L. Stibium) and atomic number 51. A metalloid, antimony has four allotropic forms. The stable form of antimony is a blue-white metal. Yellow and black antimony are unstable non-metals. Antimony is used in flame-proofing, paints, ceramics, enamels, a wide variety of alloys, electronics, and rubber.
| Contents |
Notable characteristics
Antimony in its elemental form is a silvery white, brittle, fusible, crystalline solid that exhibits poor electrical and heat conductivity properties and vaporizes at low temperatures. A metalloid, antimony, resembles metal in its appearance and physical properties, but does not chemically react as a metal. It is also attacked by oxidizing acids and halogens. Antimony and some of its alloys expand on cooling.
Estimates of the abundance of antimony in the Earth's crust range from 0.2 to 0.5 ppm. Antimony is chalcophile, occurring with sulfur and the heavy metals lead, copper, and silver.
Applications
Antimony is increasingly being used in the semiconductor industry in the production of diodes, infrared detectors, and Hall-effect devices. As an alloy, this semi-metal greatly increases lead's hardness and mechanical strength. The most important use of antimony metal is as a hardener in lead for storage batteries. Other uses;
- Batteries,
- antifriction alloys,
- type metal,
- small arms and tracer bullets,
- cable sheathing
- matches
- medicines
- plumbing ("lead-free" solder contains 5% Sb)
Antimony compounds in the form of oxides, sulfides, sodium antimonate, and antimony trichloride are used in the making of flame-proofing compounds, ceramic enamels, glass, paints, and pottery. Antimony trioxide is the most important of the antimony compounds and is primarily used in flame-retardant formulations. These flame-retardant applications include such markets as children's clothing, toys, aircraft and automobile seat covers. Also, antimony sulfide is one of the ingredients to the modern match.
History
Antimony was recognized in antiquity (3000 BC or earlier) in various compounds, and it was prized for its fine casting qualities. It was first reported scientifically by Tholden in 1450, and was known to be a metal by the beginning of the 17th century. The origin of the name "antimony" is not clear; the term may come from the Greek words "anti" and "monos", which approximately means "opposed to solitude" as it was thought never to exist in its pure form, or from the Arabian expression "Antos Ammon", which could be translated as "bloom of the god Ammon".
Antimony-symbol.png
The natural sulfide of antimony, stibnite, was known and used in Biblical times as medicine and as a cosmetic. Stibnite is still used in some developing countries as medicine. Antimony has been used for the treatment of schistosomiasis. Antimony attaches itself to sulfur atoms in certain enzymes which are used both by the parasite and human host. Small doses can kill the parasite without causing damage to the patient.
The relationship between antimony's modern name and its symbol is complex; the Coptic name for the cosmetic powder antimony sulfide was borrowed by the Greeks, which was in turn borrowed by Latin, resulting in stibium. The chemical pioneer J?Jakob Berzelius used an abbreviation of this name for antimony in his writings, and his usage became the standard symbol.
Antimony is also the first element in Tom Lehrer's "The Elements".
Sources
Even though this element is not abundant, it is found in over 100 mineral species. Antimony is sometimes found native, but more frequently it is found in the sulfide stibnite (Sb2S3) which is the predominant ore mineral. Commercial forms of antimony are generally ingots, broken pieces, granules, and cast cake. Other forms are powder, shot, and single crystals.
Precautions
Antimony and many of its compounds are toxic. Clinically, antimony poisoning is very similar to arsenic poisoning. In small doses, antimony causes headache, dizziness and depression. Such small doses have in the past been reported in some acidic fruit drinks. The acidic nature of the drink is sufficient to dissolve small amounts of antimony oxide contained in the packaging of the drink. Modern manufacturing methods prevent this occurrence. Larger doses cause violent and frequent vomiting, and will lead to death in few days.
References
- Los Alamos National Laboratory – Antimony (http://periodic.lanl.gov/elements/51.html)
- Public Health Statement for Antimony (http://www.atsdr.cdc.gov/toxprofiles/phs23.html)