Fatty acid
|
|
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid (or organic acid), often with a long aliphatic tail (long chains), either saturated or unsaturated. Most of the natural fatty acids have an even number of carbon atoms, because they are made up of acetate which has two carbon atoms.
Industrially, fatty acids are produced by the hydrolysis of the ester linkages in a fat or biological oil (both of which are triglycerides), with the removal of glycerol. See oleochemicals.
| Contents |
Saturated fatty acids
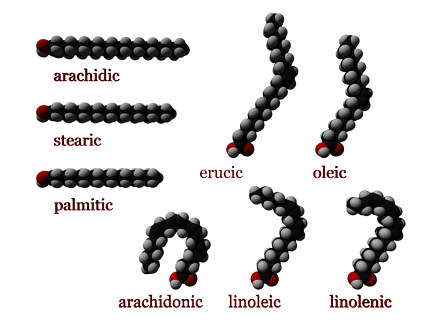
Saturated fatty acids do not contain any double bonds or other functional groups along the chain. The term "saturated" refers to hydrogen, in that all carbons (apart from the carboxylic acid [-COOH] group) contain as many hydrogens as possible. In other words, the omega (ω) end contains 3 hydrogens (CH3-) and each carbon within the chain contains 2 hydrogens (-CH2-).
Some saturated fatty acids are:
- Butyric: CH3(CH2)2COOH
- Lauric (dodecanoic acid): CH3(CH2)10COOH
- Myristic (tetradecanoic acid): CH3(CH2)12COOH
- Palmitic (hexadecanoic acid): CH3(CH2)14COOH
- Stearic (octadecanoic acid): CH3(CH2)16COOH
- Arachidic (eicosanoic acid): CH3(CH2)18COOH
Unsaturated fatty acids
Unsaturated fatty acids are of similar form, except that one or more alkene functional groups exist along the chain, with each alkene substituting a singly-bonded
-CH2-CH2-
part of the chain with a doubly-bonded
-CH=CH-
portion (that is, a carbon double bonded to another carbon). In most of these, each double bond has 3n carbon atoms after it, for some n, and are all cis bonds.
There are two different ways to make clear where these double bonds are located in the molecule. For example:
- cis/trans-Delta-x or cis/trans-Δx: The double bond is located on the xth carbon, counting down from the carboxyl terminus. The cis or trans notation indicates whether the molecule is arranged in a cis or trans conformation. In the case of a molecule having more than one double bond, the notation is, for example, cis,cis-Δ9,Δ12.
- Omega-x or ω-x : A double bond is located on the xth carbon, counting down from the distal (methyl carbon) end.
Example of unsaturated fatty acids:
- (Alpha)-Linolenic acid: CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH
- Linoleic acid: CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH
- Arachidonic acid CH3(CH2)4CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOH1 (http://webbook.nist.gov/cgi/cbook.cgi?Name=Arachidonic+Acid&Units=SI)
- Oleic acid: CH3(CH2)7CH=CH(CH2)7COOH
- Erucic acid: CH3(CH2)7CH=CH(CH2)11COOH
Linolenic acid is an omega-3 fatty acid. Linoleic acid and arachidonic acid are omega-6 fatty acids. Oleic and erucic acid are omega-9 fatty acids. Stearic and Oleic acid are both 18 C fatty acids. They differ only in that stearic acid is saturated with hydrogen, while oleic acid is an unsaturated fatty acid with two fewer hydrogen.
Essential fatty acids
Essential fatty acids are the polyunsaturated fatty acids, linoleic acid and alpha-linolenic acid, which are the parent compounds of the omega-6 and omega-3 fatty acid series respectively. They are essential in the human diet since they cannot be synthesized by the body. We can easily make saturated fatty acids and monounsaturated fatty acids with a double bond at the omega-9 poistion, but we do not have the enzymes to introduce a double bond at the omega-3 or -6 positon.
The essential fatty acids are very important for our immune system and to help us regulate our blood pressure, since they are used to make compounds such as prostaglandins. The brain is also highly enriched in derivaties of linolenic and alpha-linoleic acids.
Trans fatty acids
Main article: Trans fat
A trans fatty acid (commonly shortened to trans fat) is an unsaturated fatty acid molecule that contains a trans double bond between carbon atoms, which makes the molecule less kinked compared to fatty acids with cis double bonds. Research suggests a correlation between diets high in trans fats and diseases like atherosclerosis and coronary heart disease.
Free fatty acids
Fatty acids not bound or attached to other molecules, like triglycerides or phospholipids.
The uncombined fatty acids or free fatty acids may come from the breakdown of a triglyceride into its components (fatty acids and glycerol).
Free fatty acids are an important source of fuel for many tissues since they can yield relatively large quantities of ATP. Typically many cell types can use either glucose or fatty acids for this purpose. However, heart and skeletal muscle prefer fatty acids. On the other hand, brain cannot use fatty acids as a source of fuel, relying instead on glucose, or on ketone bodies produced by the liver from fatty acid metabolism during starvation.
pH
Formic acid and Acetic acid are totally soluble in water and dissociate to form reasonably strong acids. However, as the chain length increases the solubility of the fatty acids decreases very rapidly, and so their pH does not really have a meaning.
They will dissolve in warm ethanol, and can be titrated with sodium hydroxide solution using phenolphthalein as an indicator to a pale pink endpoint.
Autoxidation and rancidity
Fatty acids at room temperature undergo a chemical change known as autoxidation. The fatty acid breaks down into hydrocarbons, ketones, aldehydes, and smaller amounts of epoxides and alcohols. Heavy metals present at low levels in fats and oils promote autoxidation. Fats and oils often are treated with chelating agents such as citric acid.
See also
de:Fettsäuren es:Ácido graso eo:Grasacido fr:Acide gras he:חומצות שומן nl:Vetzuur ja:脂肪酸 pl:Kwas tłuszczowy sv:Fettsyra th:กรดไขมัน