Nitroglycerin
|
|
| Nitroglycerin | |
|---|---|
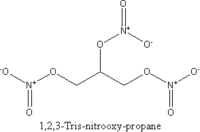
| Chemical name | 1,2,3-Tris-nitro-oxy-propane |
| Chemical formula | C3H5N3O9 |
| molecular weight | 227.0872 g/mol |
| Shock sensitivity | Very High |
| Friction sensitivity | Very High |
| Density | 1.13 at 15 °C |
| Explosive velocity | 7700 m/s |
| Boiling point | Decomposes at 50-60 °C |
| Melting point | 13.2 °C |
| Color | Yellow but colourless when pure |
| Appearance | Slightly oily liquid |
| CAS number | 55-63-0 |
| |
Nitroglycerin, also known as nitroglycerine, trinitroglycerin, and glyceryl trinitrate, is a chemical compound, a heavy, colorless, poisonous, oily, explosive liquid obtained by nitrating glycerol. It is used in the manufacture of explosives, specifically dynamite, and as such is employed in the construction and demolition industries. It is also used medically as a vasodilator to treat heart conditions. It is colored yellow when it is decomposing due to acidic pH.
| Contents |
Instability and desensitization
In its pure form, it is shock-sensitive (i.e., physical shock can cause it to explode) and degrades over time to even more unstable forms. This makes it highly dangerous to transport or use in its pure form.
Early in the history of this explosive it was discovered that liquid nitroglycerin can be "desensitized" by cooling to 5 to 10 °C (40 to 50 °F), at which temperature it freezes, contracting upon solidification. However, later thawing can be extremely sensitizing, especially if impurities are present or if warming is too rapid. It is possible to chemically "desensitize" nitroglycerin to a point where it can be considered approximately as "safe" as modern High Explosive formulations, by the addition of approximately 10 to 30 percent ethanol, acetone, or dinitrotoluene (percentage varies with the desensitizing agent used). Desensitization requires extra effort to reconstitute the "pure" product. Failing this, it must be assumed that desensitized nitroglycerin is substantially more difficult to detonate, possibly rendering it useless as an explosive for practical application.
"A serious problem in the use of nitroglycerin results from its high freezing point (13 °C [55 °F]) and the fact that the solid is even more shock-sensitive than the liquid. This disadvantage is overcome by using mixtures of nitroglycerin with other polynitrates; for example, a mixture of nitroglycerin and ethylene glycol dinitrate freezes at -29 °C (-20 °F)."1
What is detonation?
Nitroglycerin and any or all of the diluents mentioned above can certainly deflagrate, or burn. However, the explosive power of nitroglycerin is derived from detonation: a shock propagates through the fuel-rich medium at a supersonic speed. In other words, the initial burn sets up a pressure gradient that pre-ignites unshocked material, creating a fast-moving transition zone, which (due to the nature of the material) can detonate any appropriate material it encounters. This generates a self-sustained cascade of hyper-instantaneous pressure-induced combustion that grows upon itself exponentially. This is quite unlike deflagration, which depends solely upon available fuel, regardless of pressure or shock.
An explosion is essentially a very fast combustion, and combustion requires fuel and an oxidant. Nitroglycerin, as can be seen from its composition and structure (below), essentially contains both of these components. If it is detonated under pressure, it explodes to form thousands of times its original volume in hot gas.
One of these gases is nitrogen gas. N2 is very stable so its production is highly exothermic, which is why nitrogen is a main constituent of most explosives.
Preparation
- Warning!
- This chemical reaction is extremely risky to attempt except by trained professionals in specially equipped laboratories.
Nitroglycerin is prepared by nitration of glycerol (also known as glycerin). In the process, glycerin is slowly tipped into a mix of concentrated nitric and sulfuric acids. The solution is slowly mixed. The temperature should never exceed 30 °C (86 °F), otherwise there is a risk of explosion.
When the reaction is over, the mix is poured into a large amount of water. The nitroglycerin settles and is washed with water and sodium carbonate until it becomes neutral.
A book covering the in depth detailed instructions for synthesizing nitroglycerin at home is Home Workshop Explosives by Uncle Fester. The interesting thing about the synthesis is that Uncle Fester himself has personal experience in synthesizing nitroglycerin, and conveys his own detailed experiences of the synthesis in plain-written English. The release of the second edition of the book details instructions that do without the use of nitric acid. In the procedure is detailed a method which uses a mixture of sulfuric acid and potassium nitrate to create the needed nitric and sulfuric acid mixture.
Manufacturing
The industrial manufacturing process uses a 50:50 mixture of fuming sulphuric acid (fuming means it is very concentrated) and red fuming nitric acid. This produces nitronium ions in situ, which are attacked by glycerin's nucleophilic oxygen atoms. The nitro group NO2 is thus added.
The use of strong acids almost always results in an exothermic reaction (i.e., heat is produced), and this reaction is no exception. However, if the mixture becomes too hot, it explodes. Thus, the acid mixture is added slowly to the reaction vessel containing the glycerin. The reaction vessel itself is cooled with ice-cold water or some other coolant mixture at about zero °C. The vessel itself has an emergency trap door at its bottom, which hangs over a large pool of very cold water. If sensors in the mixture detect the temperature rising too rapidly, then the whole mixture can be dumped into the ice-cold water, which prevents an explosion if done in time.
Medical use
In medicine, nitroglycerin (sometimes called Glyceryl trinitrate) is used as a heart medication (under the trade names Nitrospan and Nitrostat). It is used as a medicine for angina pectoris (ischaemic heart disease) in tablets, ointment, or solution for intravenous use. A recent medical development will include a small amount of nitroglycerin in the tip of a new durex condom to stimulate erection during intercouse. "The CSD500 condom contains a chemical in its teat, called glyceryl trinitrate (GTN), which is absorbed into the skin and causes blood vessels to dilate."
The principal action of nitroglycerin is vasodilation, that is, widening of the blood vessels. The main effects of nitroglycerin in episodes of angina pectoris are
- chest pain subsides
- blood pressure decreases
- heart rate increases
These effects arise because nitroglycerin is converted to nitric oxide in the body (by a mechanism that is not completely understood), and nitric oxide in turn is a well-known natural vasodilator. Recently, it has also become popular in an off-label use at reduced (0.2%) concentration in ointment form as an effective treatment for anal fissure.
History
Nitroglycerin was discovered by Ascanio Sobrero in 1847, working under TJ Pelouze at the University of Torino. The best manufacturing process was developed by Alfred Nobel in the 1860s. His company exported a liquid combination of nitroglycerin and gunpowder as 'Swedish Blasting Oil', but the extreme danger of using the liquid, as shown in a number of "appalling catastrophes", led to the liquid being widely banned and the development of dynamite (and similar mixtures such as dualine and lithofracteur), mixing the nitroglycerine with inert (Nobel used kieselguhr) or combustible absorbents (e.g. nitrocellulose to produce the yellow gel, blasting gelatine).
External links
- Template:Web reference - 1866 Newspaper article
- Note 1: Template:Web reference
by Balthasar Dörig
bg:Нитроглицерин da:Nitroglycerin de:Glycerintrinitrat es:Nitroglicerina eo:Nitroglicerino fr:Nitroglycérine it:Nitroglicerina he:ניטרוגליצרין lv:Nitroglicerīns nl:Nitroglycerine ja:ニトログリセリン pl:Nitrogliceryna pt:Nitroglicerina sl:Nitroglicerin fi:Nitroglyseroli sv:Nitroglycerin zh:硝酸甘油
