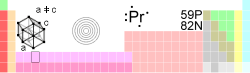
Praseodymium
|
|
| ||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | Praseodymium, Pr, 59 | |||||||||||||||||||||||||||
| Chemical series | Lanthanides | |||||||||||||||||||||||||||
| Group, Period, Block | _ , 6, f | |||||||||||||||||||||||||||
| Density, Hardness | 6640 kg/m3, no data | |||||||||||||||||||||||||||
| Appearance | silvery white, yellowish tinge
| |||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||
| Atomic weight | 140.90765(2) u | |||||||||||||||||||||||||||
| Atomic radius (calc.) | 185 (247) pm | |||||||||||||||||||||||||||
| Covalent radius | 165 pm | |||||||||||||||||||||||||||
| van der Waals radius | no data | |||||||||||||||||||||||||||
| Electron configuration | [Xe]6s24f3 | |||||||||||||||||||||||||||
| e- 's per energy level | 2, 8, 18, 21, 8, 2 | |||||||||||||||||||||||||||
| Oxidation states (Oxide) | 3 (mildly basic) | |||||||||||||||||||||||||||
| Crystal structure | hexagonal | |||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||
| State of matter | solid | |||||||||||||||||||||||||||
| Melting point | 1204 K (1707.8 ?F) | |||||||||||||||||||||||||||
| Boiling point | 3793 K (6368 ?F) | |||||||||||||||||||||||||||
| Molar volume | 20.8 ×10-6 m3/mol | |||||||||||||||||||||||||||
| Heat of vaporization | 296.8 kJ/mol | |||||||||||||||||||||||||||
| Heat of fusion | 6.89 kJ/mol | |||||||||||||||||||||||||||
| Vapor pressure | 1,333224E-06 Pa at 1070 K | |||||||||||||||||||||||||||
| Velocity of sound | 2280 m/s at 293.15 K | |||||||||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||||||||
| Electronegativity | 1.13 (Pauling scale) | |||||||||||||||||||||||||||
| Specific heat capacity | 193 J/(kg*K) | |||||||||||||||||||||||||||
| Electrical conductivity | 1.48 106/m ohm | |||||||||||||||||||||||||||
| Thermal conductivity | 12.5 W/(m*K) | |||||||||||||||||||||||||||
| 1st ionization potential | 527 kJ/mol | |||||||||||||||||||||||||||
| 2nd ionization potential | 1020 kJ/mol | |||||||||||||||||||||||||||
| 3rd ionization potential | 2086 kJ/mol | |||||||||||||||||||||||||||
| 4th ionization potential | 3761 kJ/mol | |||||||||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| SI units & STP are used except where noted. | ||||||||||||||||||||||||||||
For other meanings of the abbreviation Pr or PR, see Pr and PR.
Praseodymium is a chemical element in the periodic table that has the symbol Pr and atomic number 59.
| Contents |
Notable characteristics
Praseodymium is a soft silvery metallic element, and belongs to the lanthanide group. It is somewhat more resistant to corrosion in air than europium, lanthanum, cerium, or neodymium, but it does develop a green oxide coating that spalls off when exposed to air, exposing more metal to oxidation. For this reason, praseodymium should be stored under a light mineral oil or sealed in plastic or glass.
Applications
Uses of praseodymium:
- As an alloying agent with magnesium to create high-strength metals that are used in aircraft engines.
- Praseodymium forms the core of carbon arc lights which are used in the motion picture industry for studio lighting and projector lights.
- Praseodymium compounds are used to give glasses and enamels a yellow color.
- Praseodymium is a component of didymium glass, which is used to make certain types of welder's and glass blower's goggles.
History
The name Praseodymium comes from the Greek prasios, meaning green, and didymos, or twin.
In 1841, Mosander extracted the rare earth didymium from lanthana. In 1874, Per Teodor Cleve concluded that didymium was in fact two elements, and in 1879, Lecoq de Boisbaudran isolated a new earth, Samarium, from didymium obtained from the mineral samarskite. In 1885, the Austrian chemist baron C. F. Auer von Welsbach separated didymium into two elements, Praseodymium and Neodymium, which gave salts of different colors.
Occurrence
Praseodymium is found in the rare earth minerals monazite and bastnasite, and can be recovered from bastnasite or monazite by an ion exchange process. Praseodymium also makes up about 5% of Misch metal.
Compounds
Praseodymium compounds include:
Isotopes
Naturally occurring praseodymium is composed of one stable isotope, 141-Pr. 38 radioisotopes have been characterized with the most stable being 143-Pr with a half-life of 13.57 days and 142-Pr with a half-life of 19.12 hours. All of the remaining radioactive isotopes have half-lifes that are less than 5.985 hours and the majority of these have half lifes that are less than 33 seconds. This element also has 6 meta states with the most stable being 138m-Pr (t? 2.12 hours), 142m-Pr (t? 14.6 minutes) and 134m-Pr (t? 11 minutes).
The isotopes of praseodymium range in atomic weight from 120.955 u (121-Pr) to 158.955 u (159-Pr). The primary decay mode before the stable isotope, 141-Pr, is electron capture and the primary mode after is beta minus decay. The primary decay products before 141-Pr are element 58 (Cerium) isotopes and the primary products after are element 60 (Neodymium) isotopes.
Precautions
Like all rare earths, praseodymium is of low to moderate toxicity. Praseodymium has no known biological role.
References
- Los Alamos National Laboratory – Praseodymium (http://periodic.lanl.gov/elements/59.html)