Stimulated emission
|
|
In optics, stimulated emission is the process by which, when perturbed by a photon, matter may lose energy resulting in the creation of another photon. The perturbing photon is not destroyed in the process (cf. absorption), and the second photon is created with the same phase and frequency as the original. Stimulated emission is really a quantum mechanical phenomenon but it can be understood in terms of a classical field and a quantum mechanical atom. The process can be thought of as optical amplification, and it forms the basis of the laser and maser.
Electrons and how they interact with each other and electromagnetic fields form the basis for most of our understanding of chemistry and physics. Electrons have energy in proportion to how far they are on average from the nucleus of an atom. The Pauli exclusion principle forces some electrons to be further from the nucleus than others (that's why all the electrons don't just hang around in the 1 s orbital.) When electrons absorb energy either from light (photons) or from heat (phonons), they move further away from the atomic nuclei but they are only allowed to absorb energy that will land them into specific energy levels. This leads to emission lines and absorption lines.
When an electron is excited, it will not stay that way forever. On average there is a lifetime for any particular energy level after which half of the electrons initially in that state will have decayed into a lower state. When such a decay occurs, the energy difference between the level the electron was at and the new level must be released either as a photon or a phonon. When an electron decays due to "timeout" it is said to be due to "spontaneous emission." The phase associated with the photon that is emitted is random and has to do with some quantum mechanical ideas concerning the atom's internal state. If a bunch of electrons were put into an excited state somehow and then left to relax, the resulting radiation would be very spectrally limited (only one wavelength of light would be present) but the individual photons would not be in phase with one another. This is also called fluorescence.
Other photons (i.e. an external electromagnetic field) will affect an atom's state. The quantum mechanical variables mentioned above are changed. Specifically the atom will act like a small electric dipole which will oscillate with the external field. One of the consequences of this oscillation is it encourages electrons to decay to the lower energy state. When it does this due to the presence of other photons, the released photon is in phase with the other photons and in the same direction as the other photons. This is known as stimulated emission.
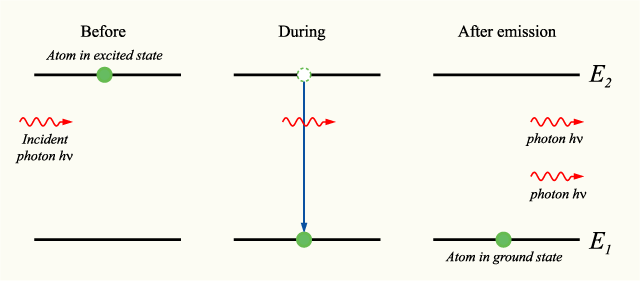
Stimulated emission can be modelled mathematically by considering an atom which may be in two electronic energy states, the ground state (1) and the excited state (2), with energies E1 and E2 respectively.
If the atom is in the excited state, it may decay into the ground state by the process of spontaneous emission, releasing the difference in energies between the two states as a photon. The photon will have frequency ν and energy hν, given by:
- <math>E_2 - E_1 = h \nu<math>,
where h is Planck's constant.
Alternatively, if the excited-state atom is perturbed by the electric field of a photon with frequency ν, it may release a second photon of the same frequency, in phase with the first photon. The atom will again decay into the ground state. This process is known as stimulated emission.
An energy level diagram illustrating the process is shown below:
In a group of such atoms, if the number of atoms in the excited state is given by N, the rate at which stimulated emission occurs is given by:
- <math>\frac{\partial N}{\partial t} = - B_{21} \rho (\nu) N <math>,
where B21 is a proportionality constant for this particular transition in this particular atom (referred to as an Einstein B co-efficient), and ρ(ν) is the radiation density of photons of frequency ν. The rate of emission is thus proportional to the number of atoms in the excited state, N, and the density of the perturbing photons.
The critical detail of stimulated emission is that the emitted photon is identical to the stimulating photon in that it has the same frequency, phase and polarisation (thus the two photons are totally coherent). It is this property that allows optical amplification to take place.
Although most directly related to the discussion of how lasers work, stimulated emission touches on some of the most basic concepts in physics and the interaction of light and matter. It is a very important and key understanding to the understanding of optics specifically and physics in general.
See also absorption, spontaneous emission, laser science, Rabi cycle.de:Induzierte Emission