Aromaticity
|
|
- This article is about a chemical property of molecules. For meanings related to odor see aroma compound.
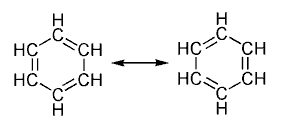
In chemistry, an aromatic molecule is one in which electrons are free to cycle around circular arrangements of atoms, which are alternately singly and doubly bonded to one another. (More properly, these bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring being identical to every other.) This commonly seen model of aromatic rings was developed by Friedrich August Kekulé von Stradonitz. The model for benzene consists of two resonance forms, which corresponds to the double and single bonds switching positions.
The two headed arrow is used in chemistry to represent resonance; it is important to realize that this does not mean that the molecule oscillates between the two states, as would be the case in an equilibrium between two different and separable products. In fact, the standard resonance representation does not in truth accurately describe a molecule; rather, it represents a possible distribution of the electronic charge. In this sense, the real molecule is a mix of these representations, or technically, a linear combination of the wavefunctions representing each electronic distribution.
A better representation is that of the circular pi bond, in which the electron density is evenly distributed through a pi bond above and below the ring. This model more correctly represents the location of electron density within the aromatic ring.
In order to be classified as aromatic, a molecular structure must satisfy these requirements:
- must have delocalized pi electron system of alternating single and double bonds
- this system must be planar
- this system must be cyclic
- the number of pi delocalized electrons in monocyclic systems must be 4n + 2, where n is a generic integer value. This is known as the Hückel rule.
Benzene, for example, satisfy all these requirements, since it has 6 delocalized pi electrons (so n = 1), whereas Cyclobutadiene does not, since the number of pi delocalized electron is 4, which is not satisfied by any n integer value.
Molecules which are not aromatic are said to be aliphatic. Aromatic molecules typically display enhanced chemical stability, compared to similar non-aromatic molecules. The circulating pi electrons in an aromatic molecule generate significant local magnetic fields that can be detected by NMR techniques.
Aromaticity was discovered by Kekulé in benzene, and was first explained in quantum mechanical terms by Linus Pauling in the 1930s. In 1931, Erich Hückel devised the "4n+2" pi electron rule, valid for planar molecules with a single ring. Molecules having 4n+2 pi electrons (n >= 0) are expected to be aromatic. Planar monocyclic molecules containing 4n pi electrons are antiaromatic.
The key aromatic hydrocarbons of commercial interest are benzene, toluene, ortho-xylene and para-xylene. About 35 million tonnes are produced worldwide every year. They are extracted from complex mixtures obtained by the refining of oil or by distillation of coal tar, and are used to produce a range of important chemicals and polymers, including styrene, phenol, aniline, polyester and nylon.
| Contents |
Examples of aromatic compounds
Heterocycles
In heterocyclic aromatics, one or more of the atoms in the aromatic ring is of an element other than carbon:
- pyridine is used as a solvent and chemical intermediate.
- furan is aromatic, but not as aromatic as benzene, and therefore is more reactive. The derivative tetrahydrofuran is very widely used as a solvent and reagent. Furan is used as a chemical intermediate. Furan is a carcinogen.
Polycyclics
Polycyclic aromatics are molecules containing two or more simple aromatic rings fused together by sharing two neighboring carbon atoms (see also simple aromatic rings).
- naphthalene
- anthracene
- phenanthrene
- polycyclic aromatic hydrocarbons (PAH)
- indole
- quinoline
- isoquinoline
Substituted aromatics
A large part of chemical compounds contain simple aromatic rings in their structure. Examples are:
- DNA (purine, pyrimidine)
- trinitrotoluene TNT (benzene)
- acetylsalicylic acid Aspirin (benzene)
- paracetamol (benzene)he:ארומטיות