Nucleophilic substitution
|
|
In chemistry, nucleophilic substitution is a type of chemical reaction in which a nucleophile electron donor replaces a leaving group as a covalent substitute of some atom. In the examples given here, this is a carbon atom, but this is far from the only possibility.
An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br, under alkaline conditions, where the attacking nucleophile is the OH- and the leaving group is Br-.
- R-Br + OH- → R-OH + Br-
In 1935, Edward D. Hughes and Sir Christopher Ingold proposed a theory proposing that there were two mechanisms at work, both of them competing with each other. The two main mechanisms of nucleophilic substitution are called SN1 and SN2. S stands for chemical substitution, N stands for nucleophilic, and the number represents the kinetic order of the reaction.
| Contents |
SN2
In the SN2 reaction, the addition of the nucleophile and the elimination of leaving group take place simultaneously somewhat similarly as in the case of the E2 mechanism.
SN2 occurs where the central carbon atom is easily accessible to the nucleophile. When the central carbon is too highly substituted, steric hindrance is so strong that SN2 is rendered close to impossible. The rate is second order, depending on both the nucleophile and the substrate concentrations. The nucleophile enters on the opposite side of the carbon to the leaving group, so a stereocenter is inverted by an SN2 reaction. Thus SN2 has the following specificities.
- It is a one-step process of substitution.
- Typical of methyl and primary substituted halides due to low steric hindrance. (Secondary substituted reacts only when with a strong nucleophile).
- The reaction rate is influenced by both the substrate and the nucleophile. :rate = k[RX][Nu-]
- Stereospecificity when the carbon is chiral.
eg. CH3Br + OH- → CH3OH + Br-
The carbon hybridization of the certain alkyl halide goes from sp3 to sp2 and then back to sp3 during the transition state. Such hybridisation draws similarity with the E2 mechanism.
SN1
SN1 tends to be important when the central carbon atom is surrounded by bulky groups, both because such groups interfere sterically with the SN2 reaction (discussed above) and because a highly substituted carbon forms a stable carbocation (the first reaction in the scheme below).
Contrary to SN2, SN1 reactions take place in two steps. The rate of the overall reaction is essentially equal to that of carbocation formation, and does not involve the attacking nucleophile. Thus the overall rate depends on the concentration of the substrate only. SN1 has the following specificities.
- It is a two-step process of substitution. The first step of carbocation formation is rate-determining
- Typical of tertiary and some secondary substituted halides due to high steric hindrance. (Secondary substituted reacts only when with a weak nucleophile)
- The reaction rate is influenced only by the substrate concentration. :rate = k[RX]
- (CH3)3CBr → (CH3)3C+ + Br-
- (CH3)3C+ + H2O → (CH3)3C-OH2+
- (CH3)3C-OH2+ + H2O → (CH3)3COH + H3O+
This gives the overall reaction
- (CH3)3C-Br + OH- → (CH3)3C-OH + Br-
Because the intermediate carbocation, R+, is planar, the central carbon is not a stereocenter, even if it was a stereocenter in the original reactant, so the original configuration at that atom is lost. Nucleophilic attack can occur from either side of the plane, so the product may consist of a mixture of two stereoisomers in the event of the molecule chirality. In fact, if the central carbon is the only stereocenter in the reaction, racemization will occur. However, an excess of inversion is usually observed, as the leaving group can remain in close proximity to the carbocation intermediate for a short time and block nucleophilic attack.
Reaction rate
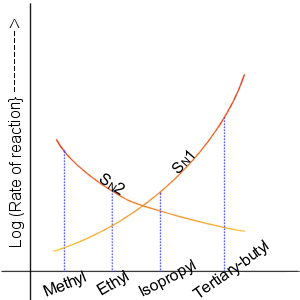
Initially, the rate of the nucleophilic substitution was a little puzzling as the rate followed the pattern :
CH3X > primary > secondary < tertiary
The reaction kinetics changed from second order to first order.
The SN1 and SN2 reactions are influenced by different factors
SN1 reactivity rates follow the trend CH3X < primary < secondary < tertiary
SN2 reactivity rates follow the trend CH3X > primary > secondary > tertiary
The total reactivity is the sum of the two rates.
A graph showing the relative reactivities of the different alkyl halides towards SN1 and SN2 reactions.
Nucleophilic aliphatic substitution reactions
- many coupling reactions
- nucleophilic substitutions can be accompanied by a allylic rearrangement for example the Ferrier rearrangement
- organic reductions with hydrides
- hydrolysis reactions
- Williamson reaction