Polonium
|
|
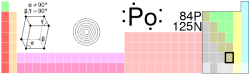
Polonium is a chemical element in the periodic table that has the symbol Po and atomic number 84. A rare radioactive metalloid, polonium is chemically similar to tellurium and bismuth and occurs in uranium ores. Polonium had been studied for possible use in heating spacecraft.
| Contents |
Notable characteristics
This radioactive substance dissolves readily in dilute acids, but is only slightly soluble in alkalis. It is closely related chemically to bismuth and tellurium. Polonium is a volatile metal with 50% being vaporized in air after 45 hours at 328 K. Polonium has no stable isotopes and has over 50 potential isotopes. Polonium is extremely toxic and highly radioactive. Polonium has been found in tobacco smoke as a contaminant and in uranium ores.
Applications
When it is mixed or alloyed with beryllium, polonium can be a neutron source. Other uses;
- This element has also been used in devices that eliminate static charges in textile mills and other places. However beta sources are more commonly used and are less dangerous.
- Polonium is used on brushes that remove accumulated dust from photographic films. The polonium in these brushes is sealed and controlled thus minimizing radiation hazards.
Polonium-210
This isotope of polonium is an alpha emitter that has a half-life of 138.39 days. A milligram of this metalloid emits as many alpha particles as 5 grams of radium. A great deal of energy is released by its decay with a half a gram quickly reaching a temperature above 750 K. A few curies (gigabecquerels)of polonium-210 emit a blue glow which is caused by excitation of surrounding air. A single gram of polonium-210 generates 140 watts of heat energy. Since nearly all alpha radiation can be easily stopped by ordinary containers and upon hitting its surface releases its energy, Polonium-210 has been used as a lightweight heat source to power thermoelectric cells in artificial satellites. Because of its short halflife though polonium-210 cannot provide power for long-term space missions and has been phased out of use in this application.
History
Also called Radium F, polonium was discovered by Marie and Pierre Curie in 1898 and was later named after Marie's home land of Poland. Poland at the time was under Russian, Prussian and Austrian domination, and not recognized as an independent country. It was Marie's hope that naming the element after her home land would add notoriety to its plight. Polonium may be the first element named to highlight a political controversy.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity. The pitchblende, after removal of uranium and radium, was more radioactive than both radium and uranium put together. This spurred them on to find the element. The electroscope showed it separating from bismuth.
Occurrence
A very rare element in nature, polonium is found in uranium ores at about 100 micrograms per metric ton (1:1010). Its natural abundance is approximately 0.2% of radium's.
In 1934 an experiment showed that when natural bismuth (Bi-209) is bombarded with neutrons, Bi-210, which is the parent of polonium, was created. Polonium may now be made in milligram amounts in this procedure which uses high neutron fluxes found in nuclear reactors.
Isotopes
Polonium has many isotopes all of which are radioactive. There are 25 known isotopes of polonium with atomic masses that range from 194 u to 218 u. Polonium-210 is the most widely available. Po-209 (half-life 103 years) and Po-208 (half-life 2.9 years) can be made through the alpha, proton, or deuteron bombardment of lead or bismuth in a cyclotron. However these isotopes are expensive to produce.
Precautions
Polonium is a highly radioactive and toxic element and is dangerous to handle. Even milligram or microgram amounts, handling polonium-210 is very dangerous and requires special equipment used with strict procedures. Direct damage occurs from energy absorption into tissues from alpha particles.
The maximum allowable body burden for ingested polonium is only 1100 becquerels (0.03 microcurie), which is equivalent to a particle weighing only 6.8 x 10-12 gram. Weight for weight polonium is approximately 2.5 x 1011 times as toxic as hydrocyanic acid. The maximum permissible concentration for airborne soluble polonium compounds is about 7,500 Bq/m³ (2 x 10-11 ?Ci/cm3).
References
- Los Alamos National Laboratory – Polonium (http://periodic.lanl.gov/elements/84.html)