Electron
|
|
| Electron | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
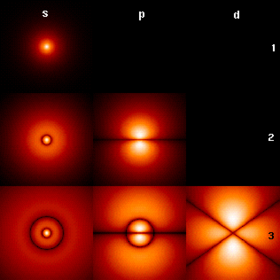 The first few hydrogen atom electron orbitals shown as cross-sections with color-coded probability density | ||||||||||||||
| Classification | ||||||||||||||
| ||||||||||||||
| Properties | ||||||||||||||
|
The electron is a subatomic particle. In an atom the electrons surround the nucleus of protons and neutrons in an electron configuration. The word electron was coined in 1894 and is derived from the term electric, whose ultimate origin is the Greek word meaning amber]. Electrostatic charge can be generated by rubbing the amber with the pelt of an animal e.g. a cat and has been done so while analysing elementary charge for the first time. The ending -on, shared by most subatomic particles, was used in analogy to the word ion.
As stated above, electrons have an electrical charge. When they move, they generate an electric current. Because the electrons of an atom determine the way in which it interacts with other atoms, they confer chemical properties to elements and therefore play a fundamental part in chemistry.
| Contents |
Electrons in practice
Classification of electrons
The electron is one of a class of subatomic particles called leptons which are believed to be fundamental particles (that is, they cannot be broken down into smaller constituent parts). The word "particle" is somewhat misleading however, because quantum mechanics shows that electrons also behave like a wave.
Properties and behavior of electrons
The term electron usually refers to negatrons with a negative electric charge of −1.6 צamp;nbsp;10−19 coulombs, and a mass of about 9.11 צamp;nbsp;10−31 kg (0.51 MeV), which is approximately 1⁄1836 of the mass of the proton. These are commonly represented as e−. Sometimes the term is used, as proposed by Carl D. Anderson, to refer to both negatrons and positrons. Positrons have the same mass and an electric charge of the equal but positive value.
The motion of the electron about the nucleus is a somewhat controversial topic. The electron does not exhibit motion in the physical sense — it does not "float"; rather, it seems to appear in and out of existence, at various points around the nucleus (of course, 90% of the time the electron can be found in its designated orbital). A simple analogy would be a firefly, in a dark room, lighting up at various points about a central light source — it can light up anywhere, but it is most likely to appear closer to the source than otherwise. At present, we cannot predict both the momentum and position of an electron. This is a limitation described by the Heisenberg uncertainty principle, which, simplified and tailored for quantum particles, simply states that the more accurately we know a particle's position, the less accurately we can know its momentum and vice versa.
The electron has spin ?, which implies it is a fermion, i.e., it follows the Fermi-Dirac statistics. While most electrons are found in atoms, others move independently in matter, or together as an electron beam in a vacuum. In some superconductors, electrons move in "Cooper pairs," in which their motion is coupled to nearby matter via lattice vibrations called phonons. When electrons move, free of the nuclei of atoms, and there is a net flow, this flow is called electricity, or an electric current.
So-called "static electricity" is not a flow of electrons. More correctly called a "static charge", it refers to a body that has more or fewer electrons than are required to balance the positive charge of the nuclei. When there is an excess of electrons, the object is said to be "negatively charged". When there are fewer electrons than protons, the object is said to be "positively charged". When the number of electrons and the number of protons are equal, the object is said to be electrically "neutral". Electrons and positrons can annihilate each other and produce a photon. Conversely, a high-energy photon can be transformed into an electron and a positron by a process called pair production.
The electron is an elementary particle – that means that it has no substructure (at least, experiments have not found any so far, and there is good reason to believe that there is not any). Hence, it is usually described as point-like, i.e. with no spatial extension. (However, if one gets very near an electron, one notices that its properties (charge and mass) seem to change. This is an effect common to all elementary particles: the particle influences the vacuum fluctuations in its vicinity, so that the properties one observes from far away are the sum of the bare properties and the vacuum effects – see renormalization.)
There is a physical constant called the classical electron radius, with a value of 2.8179 צamp;nbsp;10−15 m. Note that this is the radius that one could infer from its charge if the physics were only described by the classical theory of electrodynamics and there were no quantum mechanics (hence, it is an outdated concept that nevertheless sometimes still proves useful in calculations). The speed of an electron in a vacuum aproaches but never reaches c, the speed of light in a vacuum. This is due to an effect of special relativity. The effects of special relativity are based on a quantity known as gamma or the Lorentz factor.
Electrons in the universe
It is believed that the number of electrons existing in the known universe is at least 1079. This number amounts to a density of about one electron per cubic metre of space.
Based on the classical electron radius and assuming a dense sphere packing, it can be calculated that the number of electrons that would fit in the observable universe is on the order of 10130. Of course, this number is even less meaningful than the classical electron radius itself.
Electrons in industry
Electron beams are used in welding as well as lithography.
Electrons in the laboratory
Founding experiments
The quantum or discrete nature of electron's charge was observed by Robert Millikan in the Oil-drop experiment of 1909.
Use of electrons in the laboratory
Electron microscopes are used to magnify details up to 500,000 times. Quantum effects of electrons are used in Scanning tunneling microscope to study features at the atomic scale.
Electrons in theory
In quantum mechanics, the electron is described by the Dirac Equation. In the Standard Model of particle physics, it forms a doublet in SU(2) with the electron neutrino, as they interact through the weak interaction. The electron has two more massive partners, with the same charge but different masses: the muon and the tauon.
The antimatter counterpart of the electron is its antiparticle, the positron. The positron has the same amount of electrical charge as the electron, except that the charge is positive. It has the same mass and spin as the electron. When an electron and a positron meet, they may annihilate each other, giving rise to two gamma-ray photons, each having an energy of 0.511 MeV (511 keV). See also Electron-positron annihilation.
Electrons are also a key element in electromagnetism, an approximate theory that is adequate for macroscopic systems.
History
The electron as a unit of charge in electrochemistry had been posited by G. Johnstone Stoney in 1874. In 1894, he also invented the word itself.
The discovery that the electron was a subatomic particle was made in 1897 by J.J. Thomson at the Cavendish Laboratory at Cambridge University, while he was studying "cathode rays". Influenced by the work of James Clerk Maxwell, and the discovery of the X-ray, he deduced that cathode rays existed and were negatively charged "particles", which he called "corpuscles". He published his discovery in 1897.
The periodic law states that the chemical properties of elements largely repeat themselves periodically and is the foundation of the periodic table of elements. The law itself was initially explained by the atomic mass of the elements. However, as there were anomalies in the periodic table, efforts were made to find a better explanation for it. In 1913, Henry Moseleyintroduced the concept of the atomic number and explained the periodic law with the number of protons each element has. In the same year, Niels Bohr showed that electrons are the actual foundation of the table. In 1916, Gilbert Newton Lewis and Irving Langmuir explained the chemical bonding of elements by electronic interactions. Template:Chem clipart