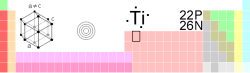
Titanium
|
|
| |||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | Titanium, Ti, 22 | ||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 4, 4, d | ||||||||||||||||||||||||||||||||||||||||||
| Density, Hardness | 4507 kg/m3, 6 | ||||||||||||||||||||||||||||||||||||||||||
| Appearance | Silvery metallic Missing image Ti,22.jpg | ||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||
| Atomic weight | 47.867 amu | ||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 140 (176) pm | ||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 136 pm | ||||||||||||||||||||||||||||||||||||||||||
| van der Waals radius | no data | ||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar]3d2 4s2 | ||||||||||||||||||||||||||||||||||||||||||
| e-'s per energy level | 2, 8, 10, 2 | ||||||||||||||||||||||||||||||||||||||||||
| Oxidation state (Oxide) | 4 (amphoteric) | ||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | Hexagonal | ||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||
| State of matter | Solid (__) | ||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1941 K (3034 ?F) | ||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3560 K (5949 ?F) | ||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 10.64 ×10-6 m3/mol | ||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 421 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 15.45 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | 0.49 Pa at 1933 K | ||||||||||||||||||||||||||||||||||||||||||
| Velocity of sound | 4140 m/s at 293.15 K | ||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.54 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 520 J/(kg*K) | ||||||||||||||||||||||||||||||||||||||||||
| Electrical conductivity | 2.34 106 S/m | ||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 21.9 W/(m*K) | ||||||||||||||||||||||||||||||||||||||||||
| 1st ionization potential | 658.8 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 2nd ionization potential | 1309.8 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 3rd ionization potential | 2652.5 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 4th ionization potential | 4174.6 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 5th ionization potential | 9581 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 6th ionization potential | 11533 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 7th ionization potential | 13590 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 8th ionization potential | 16440 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 9th ionization potential | 18530 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 10th ionization potential | 20833 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||
| SI units & STP are used except where noted. | |||||||||||||||||||||||||||||||||||||||||||
Titanium is a chemical element in the periodic table that has the symbol Ti and atomic number 22. It is a light, strong, lustrous, corrosion-resistant (including resistance to sea water and chlorine) transition metal with a white-silvery-metallic color. Titanium is used in strong light-weight alloys (most notably with iron and aluminum) and its most common compound, titanium dioxide, is used in white pigments.
This element occurs in numerous minerals with the main sources being rutile and ilmenite, which are widely distributed over the Earth. There are two allotropic forms and five naturally-occurring isotopes of this element; Ti-46 through Ti-50 with Ti-48 being the most abundant (73.8%). One of titanium's most notable characteristics is that it is as strong as steel but is less than half its weight. Titanium's properties are chemically and physically similar to zirconium.
| Contents |
Notable characteristics
Titanium is well known for its excellent corrosion resistance (almost as resistant as platinum), being able to withstand attack by acids, moist chlorine gas, and by common salt solutions. Pure titanium is not soluble in water but is soluble in concentrated acids. A metallic element, it is also well-known for its high strength-to-weight ratio. It is a light, strong metal with low density (40% as dense as steel) that, when pure, is quite ductile (especially in an oxygen-free environment), easy to work, lustrous, and metallic-white in color. The relatively high melting point of this element makes it useful as a refractory metal. Titanium is as strong as steel, but 45% lighter; it is 60% heavier than aluminum, but twice as strong. These properties make titanium very resistant to the usual kinds of metal fatigue.
This metal forms a passive but protective oxide coating (leading to corrosion-resistance) when exposed to elevated temperatures in air but at room temperatures it resists tarnishing. The metal, which burns when heated in air 610° C or higher (forming titanium dioxide) is also one of the only elements that burn in pure nitrogen gas (it burns at 800° C and forms titanium nitride). Titanium is resistant to dilute sulfuric and hydrochloric acid, along with chlorine gas, chloride solutions, and most organic acids. It is paramagnetic (weakly attracted to magnets) and has a very low electrical and thermal conductivity.
Experiments have shown that natural titanium becomes very radioactive after it is bombarded with deuterons, emitting mainly positrons and hard gamma rays. The metal is a dimorphic allotrope with the hexagonal alpha form changing into the cubic beta form very slowly at around 880°C. When it is red hot the metal combines with oxygen, and when it reaches 550°C it combines with chlorine. It also reacts with the other halogens and absorbs hydrogen.
Applications
Approximately 95% of titanium is consumed in the form of titanium dioxide (TiO2), an intensely white permanent pigment with good covering power in paints, paper, and plastics. Paints made with titanium dioxide are excellent reflectors of infrared radiation and are therefore used extensively by astronomers and in exterior paints. It is also used as a strengthening filler in paper and cement and in gemstones.
Because of its very high tensile strength (even at high temperatures), light weight, extraordinary corrosion resistance, and ability to withstand extreme temperatures, titanium alloys are principally used in aircraft, armor plating, naval ships, spacecraft and missiles. It is used in steel alloys to reduce grain size and as a deoxidizer but in stainless steel it is employed to reduce carbon content. Titanium is often alloyed with aluminium (to refine grain size), vanadium, copper (to harden), iron, manganese, molybdenum and with other metals.
Its vanadium alloy is used to make the outer skin of aircraft, to make firewalls, landing gear, and hydraulic tubing. A typical commercial jet airplane contains 318 to 1134 kg (700 and 2500 lb) of titanium. Use of titanium in consumer products such as golf clubs, bicycles, laboratory equipment, wedding bands, and laptop computers is becoming more common.
Other uses:
- Due to excellent resistance to sea water, it is used to make propeller shafts and rigging and in the heat exchangers of desalination plants.
- It is used to produce relatively soft artificial gemstones.
- Titanium tetrachloride (TiCl4), a colorless liquid, is used to iridize glass and because it fumes strongly in moist air it is also used to make smoke screens and in skywriting.
- In addition to being a very important pigment, titanium dioxide is also used in sunscreens due to its ability to protect skin by itself.
- Because it is considered to be physiologically inert, the metal is used in joint replacement implants such as hip ball and sockets and to make medical equipment and in pipe/tank lining in food processing.
- Its inertness and ability to be attractively colored makes it a popular metal for use in body piercing.
- Titanium has the unusual ability to osseointegrate, enabling use in dental implants.
Titanium has occasionally been used in construction: the 150-foot memorial to Yuri Gagarin, the first man to travel in space, in Moscow, is made of titanium for the metal's attractive color and association with rocketry. The Guggenheim Museum Bilbao and the Cerritos Library were the first buildings, respectively, in Europe and North America to be sheathed in titanium panels.
History
Titanium (Latin Titans, Earth or the first sons of Gaia) was discovered in England by Reverend William Gregor in 1791. He recognized the presence of a new element in ilmenite, and named it menachite. At around the same time, Franz Joseph Muller also produced a similar substance, but could not identify it. The element was independently rediscovered several years later by German chemist Martin Heinrich Klaproth in rutile ore. Klaproth confirmed it as a new element and in 1795 he named it for the Latin word for Earth (also the name for the Titans of Greek mythology).
The metal has always been difficult to extract from its various ores. Pure metallic titanium (99.9%) was first prepared in 1910 by Matthew A. Hunter by heating TiCl4 with sodium in a steel bomb at 700-800°C in the Hunter process. Titanium metal was not used outside the laboratory until 1946 when William Justin Kroll proved that titanium could be commercially produced by reducing titanium tetrachloride with magnesium in the Kroll process which is the method still used today.
In 1950-1960s the Soviet Union attempted to corner the world titanium market as a tactic in the Cold War to prevent the American military from utilizing it. In spite of these efforts, the U.S obtained large quantities of titanium when a European company set up a front for the US foreign intelligence agencies to purchase it.
Occurrence and production
Titanium metal is not found unbound to other elements in nature but the element is the ninth most abundant element in the Earth's crust (0.6% by mass) and is present in most igneous rocks and in sediments derived from them (as well as in living things and natural bodies of water). It is widely-distributed and occurs primarily in the minerals anatase, brookite, ilmenite, perovskite, rutile, titanite (sphene), as well in many iron ores. Of these minerals, only ilmenite and rutile have significant economic importance, yet even they are difficult to find in high concentrations. Because it reacts easily with oxygen and carbon at high temperatures it is difficult to prepare pure titanium metal, crystals, or powder. Significant titanium ore deposits are in Australia, Scandinavia, North America and Malaysia.
This metal is found in meteorites and has been detected in the sun and in M-type stars. Rocks brought back from the moon during the Apollo 17 mission are composed of 12.1% TiO2. Titanium is also found in coal ash, plants, and even the human body.
TitaniumUSGOV.jpg
Because the metal reacts with air at high temperatures it can not be produced by reduction of its dioxide. Titanium metal is therefore produced commercially by the Kroll process; a complex, and expensive batch process developed in 1946 by William Justin Kroll. In the Kroll process chlorine gas is passed over red-hot rutile or ilmenite in the presence of carbon to make TiCl4. This is condensed and purified by fractional distillation and then reduced with 800°C molten magnesium in an argon atmosphere.
A newer process called the FFC Cambridge Process may displace this older process. This method uses the feedstock titanium dioxide powder (which is a refined form of rutile) to make the end product which is either a powder or sponge. If mixed oxide powders are used, the product is an alloy at a much lower cost than the conventional multi-step melting process. It is hoped that the FFC Cambridge Process will render titanium a less rare and expensive material for the aerospace industry and the luxury goods market, and will be seen in many products currently manufactured using aluminum and specialist grades of steel.
Titanium oxide is produced commercially by grinding its mineral ore and mixing it with potassium carbonate and aqueous hydrofluoric acid. This yields potassium fluorotitanate (K2TiF6) which is extracted with hot water and decomposed with ammonia, producing a ammoniacal hydrated oxide. This in turn is ignited in a platinum vessel, which creates pure titanium dioxide.
Common titanium alloys are made by reduction. For example; cuprotitanium (rutile with copper added is reduced), ferrocarbon titanium (ilmenite reduced with coke in an electric furnace), and manganotitanium (rutile with manganese or manganese oxides are reduced).
Compounds
The +4 oxidation state dominates in titanium chemistry, but compounds in the +3 oxidation state are also common. Because of this high oxidation state, many titanium compounds have a high degree of covalent bonding.
Although titanium metal is relatively uncommon, due to the cost of extraction, titanium dioxide (also called titanium(IV), titanium white, or even titania) is cheap, nontoxic, readily available in bulk, and very widely used as a white pigment in paint, enamel, lacquer, plastic and construction cement. TiO2 powder is chemically inert, resists fading in sunlight, and is very opaque: this allows it to impart a pure and brilliant white color to the brown or gray chemicals that form the majority of household plastics. In nature, this compound is found in the minerals anatase, brookite, and rutile.
Paint made with titanium dioxide does well in severe temperatures, is somewhat self-cleaning, and stands up to marine environments. Pure titanium dioxide has a very high index of refraction and an optical dispersion higher than diamond. Star sapphires and rubies get their asterism from the titanium dioxide present in them. Titanates are compounds made with titanium dioxide. Barium titanate has piezoelectric properties, thus making it possible to use it as a transducer in the interconversion of sound and electricity. Esters of titanium are formed by the reaction of alcohols and titanium tetrachloride and are used to waterproof fabrics.
Titanium(IV) chloride (titanium tetrachloride, TiCl4, sometimes called "Tickle") is a colorless, weakly acidic liquid which is used as an intermediate in the manufacture of titanium(IV) oxide for paint. It is widely used in organic chemistry as a Lewis acid, for example in the Mukaiyama aldol condensation. Titanium also forms a lower chloride, titanium(III) chloride (TiCl3), which is used as a reducing agent.
Titanocene dichloride is an important catalyst for carbon-carbon bond formation. Titanium isopropoxide is used for Sharpless epoxidation. Other compounds include; Titanium bromide (used in metallurgy, superalloys, and high-temperature electrical wiring and coatings) and titanium carbide (found in high-temperature cutting tools and coatings).
Isotopes
Naturally occurring titanium is composed of 5 stable isotopes; Ti-46, Ti-47, Ti-48, Ti-49 and Ti-50 with Ti-48 being the most abundant (73.8% natural abundance). Eleven radioisotopes have been characterized with the most stable being Ti-44 with a half-life of 63 years, Ti-45 with a half-life of 184.8 minutes, Ti-51 with a half-life of 5.76 minutes, and Ti-52 with a half-life of 1.7 minutes. All of the remaining radioactive isotopes have half-lifes that are less than 33 seconds and the majority of these have half lifes that are less than half a second.
The isotopes of titanium range in atomic weight from 39.99 amu (Ti-40) to 57.966 amu (Ti-58). The primary decay mode before the most abundant stable isotope, Ti-48, is electron capture and the primary mode after is beta emission. The primary decay products before Ti-48 are element 21 (scandium) isotopes and the primary products after are element 23 (vanadium) isotopes.
Precautions
When in a metallic powdered form, titanium metal poses a significant fire hazard and, when heated in air, an explosion hazard. Water and carbon dioxide-based methods to extinguish fires are ineffective on burning titanium; sand, dirt, or special foams must be used instead. Salts of titanium are often considered to be relatively harmless but chlorine compounds, such as TiCl3 and TiCl4, should be considered corrosive. Titanium also has a tendency to bio-accumulate in tissues that contain silica but it does not play any known biological role in humans.